Health Data
In the healthcare sector, data is a key element. The advent of digital technology opens up new opportunities to collect and store a wealth of information.
Unfortunately, more and more data does not mean better quality or better treatments. This is why KYomed INNOV proposes to accompany you in this universe of health data.
In digital health, this data is therefore collected through sensors, connected medical devices, applications or questionnaires, which then need to be processed and used.
We accompany you in this analysis and the valorisation of your data in order to meet your needs in terms of :

Clinical studies

Scientific research

Use of your digital health solution

Development of algorithms or artificial intelligence (A.I.)
The exponential growth of digital data also creates a need for anticipation. Indeed, the design and management of a health database must be based on a reliable structure that complies with current standards. This requires anticipating this structuring upstream of data collection, then constantly monitoring its evolution.
For this mission, our team and our partners accompany you in the management of your data. Because security is one of our priorities, we also ensure that it is consistent with the regulations while making it usable for a purpose of use, marketing or clinical study.
Health Data Analysis
Statistical analysis is the only way to interpret health data and identify their added value in a meaningful way.
Together, and according to your objectives, we adapt our biostatistical, biomathematical or bioinformatics methods to enable you to :

Describe your data

Identify significant differences in your sample

Evaluate the predictive power of your data/marker combinations

Evaluate the diagnostic performance of your markers of interest (sensitivity and specificity)

Analyze concordance

Analyze survival

Identify risk and protective factors in the population of interest (epidemiology)

Classify your data

Identify and separate clusters/groups of individuals

Model a phenomenon of interest (algorithmic)

Build an algorithm or an artificial intelligence

…
Valuation of your data
KYomed INNOV and its team can assist you in making the most of your data at several levels:

Internally, to better understand how your customers use your solution in order to improve it;

For your users to improve the perceived value of your solution to them;

Externally, with actors potentially interested in the data you generate.
Thanks to our mastery of databases and statistics, we work with the data generated by your healthcare solution to make it as useful and relevant as possible.
Whether the data comes from connected devices, sensors or even algorithms or Artificial Intelligence (A.I.), we support you in the process of structuring and valuing them.
The data generated around your solution can initially be used internally.
Data analysis in the context of a scientific research or an innovative project
In the context of scientific research on patients, the data collected to follow the evolution of a pathology, or the impact of a therapy may at first appear to be uncorrelated. A thorough analysis is then necessary to try to combine some of the measured parameters and identify markers correlated with the evolution of the pathology for example.
Attention, to comply with GDPR, it is necessary to justify the collection of data, to describe the use that will be made of it and to optimize the volume of data collected. It is not possible to collect data without objective and justification.
This type of analysis allows, once the data of interest have been collected, to consider several parameters and to test combinations to identify and select relevant and specific health markers. This combination of markers is called a pathology signature.

The statistician will use the most appropriate biomathematical, biostatistical and bioinformatics methods to integrate and analyze the collected health data in a univariate or combinatorial way, to identify possible combinations of health markers.

The best combinations will be selected based on statistical performance, clinical relevance and practicality, taking into account feasibility in the field.

Once selected, these markers will go through an analytical validation step that consists of providing solid evidence of specificity, sensitivity, robustness, detection limits, accuracy, repeatability, and reproducibility of the analytical procedure.
Statistical analysis of data in a clinical study
Data valorization through statistical analysis is also implemented in the framework of a clinical study.
Indeed, before the realization of a clinical investigation, we choose and build together the methodology and the design of your study. This methodology is established to find the best possible way to valorize your solution and its impact through the collected data.
In this essential phase, the statistician intervenes to:

Determine the number of subjects needed (NSN)

Write the statistical analysis plan
Then, following the realization of the clinical investigation and thus the collection of data as well as the data management, comes the stage of statistical analysis. This analysis is carried out according to the statistical analysis plan established in the study methodology and written in the protocol.
Within this regulated framework, the analysis cannot differ from what was chosen in the upstream phase. If the results are not significant and therefore do not validate the objectives of the study, unfortunately nothing can be changed. This is why the methodology and design phase of the study is essential.

Deliverable :
Following this analysis, a statistical report is written and integrated into the clinical report.
Data analysis when using a digital health solution
Thanks to the use of your solution, data are brought back to you:

By your solution directly, via usage statistics (time spent on the solution, on a screen, user path, interaction with buttons, …)

By your users, in the feedback they can give you
This user experience data is very rich and can bring a lot to the table if it is properly processed, investigated, and implemented. The main objective of processing this data is to improve the experience of your users when using your solution. This may involve:
Understanding the solution and how it works
The logic of the user path
Interactions with the different CTAs (Call To Action)
We can help you in these steps by exploiting this UX data to improve the adherence and acceptability of your solution by your users. We can also work with you on optimizing the data collected and the questions asked in order to qualify the data collection on your solution.
The data reported by your users can be an important source of ideas. It can allow you to work and increase the added value for your users and thus the potential of your solution. We accompany you in this analysis of your data to identify the keys to optimization and their power of innovation.
Use of your data for your users

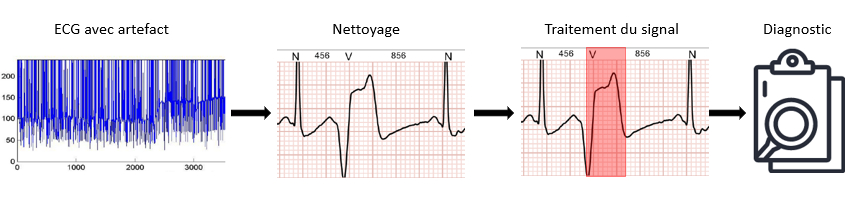
The processing of your data can take place at several levels. You can decide to provide your users with what is called “raw data“, for example when you present an electrocardiogram to a cardiologist. He knows how to read and interpret it. In this case your perceived value remains limited.

If you decide to integrate an algorithm or an artificial intelligence to this treatment then you will be able to pre-detect heart rhythm pathologies. This second level allows the cardiologist to save time and to focus on and validate only the problematic segments of his recording. Your perceived value starts to become important.

If you then decide toaggregate different factors in addition to the ECG you may be able to build new health markers to be protected by intellectual property and that will allow your users not only to save time but also to have simplified diagnoses or an aggregation of relevant data to refine their diagnosis.
UI Visualization and Combining New Markers
Data visualization is playing an increasingly important role in helping us make sense of the billions of rows of data generated every day. Our guidance will help you highlight trends and unusual values while eliminating irrelevant ones.
By presenting only useful data, we will help you increase the perceived value of your solution and thus its adoption by future users.
This involves:

Identifying discriminating health markers

Identifying the best performing combinations of markers
The data collected by your solution and its users can have a potential with various and varied actors (insurance companies, mutual insurance companies, pharmaceutical laboratories, research units, industrialists, …). Today, data has an important value.
This reflection must start at the beginning of your project to imagine the future uses of the data you will collect. We can assist you in this phase of identifying potential customers or partners for whom your data would be valuable.
It is also crucial to structure your data well from the beginning to ensure that it will be usable and valuable to your various targets.
You also need to ensure that this data is qualitative and quantitative so that it is of interest to other stakeholders.
Path of your data
Regulations should not be a barrier to the development of your solution but, on the contrary, a force to convince of the reliability of your project. Securing your data also means protecting your future users by respecting their privacy.
In the context of health data, regulations are very strict and we help you stay within an ethical and regulatory framework on the use of your data while maximizing its value.
The subject of health data is increasingly sensitive among the population, so it is very important to match your general conditions of use, the communication around your product, and potentially an ethics committee so that your users are aware of the use that will be made of their data or the data they generate. The processing of health and personal data falls within various national and international regulatory frameworks such as :

The CNIL

The GDPR “General Data Protection Regulation”

The HDH certification “Health Data Hosting”
This requires great vigilance in all phases of the life and use of your data.
With our team of experts, we can accompany you in each of these phases to ensure that the operation of your solution will be in perfect adequacy with the local and/or international regulations.










In the context of a medical device, when acquiring your data, you must ensure, for example, that the person providing the data is identified, that the data collected is complete and that the user has a good understanding of the use that can be made of each data generated or provided.
The CNIL is particularly vigilant in France about the collection of data that are considered unnecessary. We therefore help you in the reflection of the use that will be made of these data to justify this acquisition.
Once your data is acquired, it may have to be transferred between different connected objects and between different servers. Depending on the nature of the technologies used (Bluetooth, Sigfox, wifi network) technical protection solutions will have to be put in place to ensure that it will not be possible or at least possible to hack the data during their transfer.
From the implementation of an efficient solution to a blockchain and encryption, we can help you make choices that are both user-oriented and data protection-oriented.
When transferred, the data will then have to be stored either on mobile devices or on servers. Depending on the categorization and type of your data, these storage spaces will have to comply with French regulations.
Your data may even have to be hosted on specific servers named HDS (Health Data Host). The choice of your provider for this HDS hosting will also have an impact on the credibility of your solution and on the image that your company may have with its future customers.
HDS solutions are currently available from large companies such as Microsoft or Amazon but also from French or European operators such as Claranet or OVH. A list of these certified hosting companies is maintained by the government and presents the classification levels for which each of these hosting companies is certified. There are 6 levels of key activities divided into 2 certificates.
Again, depending on your data and your needs, we will help you select the right level of activity to optimize your hosting costs.
This phase is not mandatory depending on the uses of your data.
Anonymization is a technique that removes all identifying information from a data set. According to the ISO 29100 standard, it is “the process by which personally identifiable information (PII) is irreversibly altered”. Anonymization is therefore marked by the irreversible loss of the identifiable character of individuals.
In contrast, pseudonymization or reversible anonymization consists of replacing one attribute by another in a record. The physical person is thus always likely to be identified indirectly.
Each data has a lifespan, so it is important to define them well in order not to saturate the servers from an ecological point of view and also to optimize your costs, because hosting health data is particularly expensive.
It is therefore important to have a strategy on the conservation of data according to the regulations in force and according to the needs of use that you can make of it. The European regulation RGPD also requires you to have a traceability on the destruction of these data especially if they come from your users.
In collaboration with our IT partners specialized in health data, the KYomed INNOV team will accompany you and help you optimize your data flow.
Contact us
If you have a digital health project, our entire team is here to help you.
By submitting this form, I agree to the use of my personal information to contact me. I also accept the privacy policy.